Your cart is currently empty!
Truvada vs Descovy: Why the Switch?
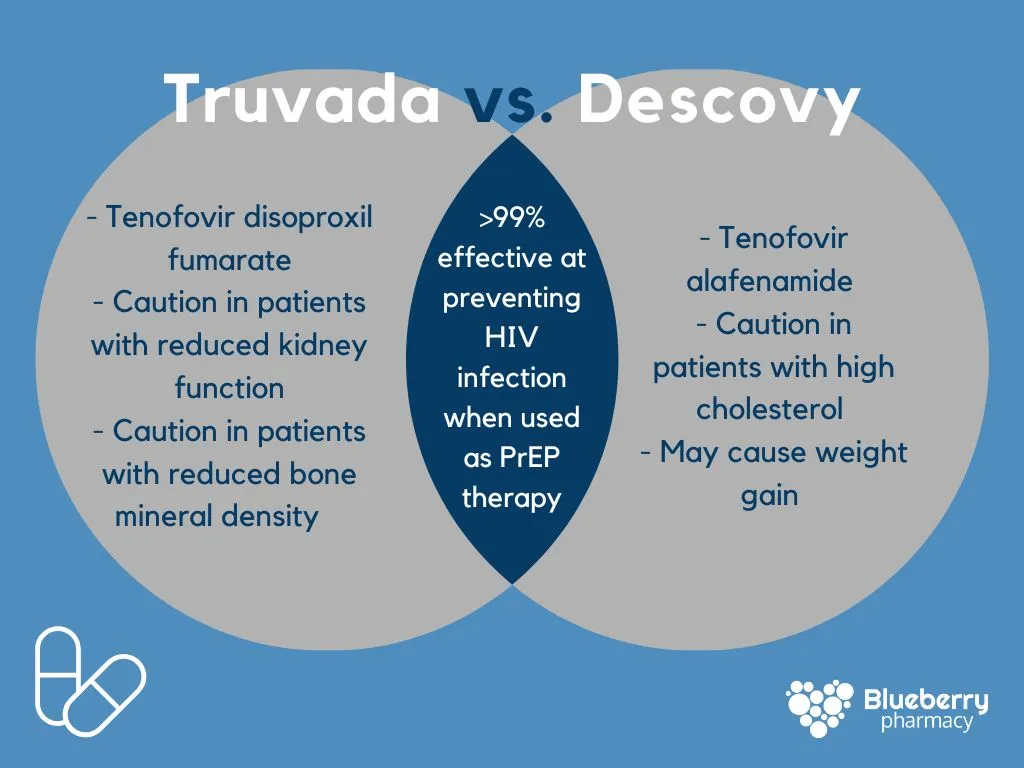
Truvada is a brand-name HIV nucleoside analog reverse transcriptase inhibitor (NRTI), used to prevent and treat HIV infection. It is made up of a combination of two drugs, emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF). Descovy is also a brand-name HIV nucleoside analog reverse transcriptase inhibitor (NRTI), with similar active ingredients as Truvada that include both emtricitabine (FTC) and tenofovir alafenamide (TAF). Both of these drugs are most commonly used for pre-exposure prophylaxis therapy (PrEP), for those who are at-risk of contracting HIV through sexual intercourse. While both of these medications are manufactured by Gilead and have similar efficacy rates at preventing HIV infection, many patients are being switched from Truvada to Descovy — but the question is why?
Truvada became available as a generic medication in March of 2021, while Descovy will remain a brand-name medication until Gilead’s patent on the drug expires in 2032. When Descovy was FDA approved in late 2019, a study found that 29% of patients switched from Truvada to Descovy during the first 6 months of approval — with many of these switches having no clinical reasoning. Because each of these medications has a different formulation of tenofovir, different side effects have been reported as noted in the graphic above. These side effects may be a reason for some patients’ moves to Descovy, but many of these switches are not relevant to the patient and may just be inflating costs to the healthcare system. Now that Truvada is available in a generic formulation, changing patients to Descovy may be leading to higher copays, out of pocket costs, and overall no significant difference in HIV infection prevention and side effects. In 2020, Medicare spent over $500,000,000 on Descovy, with an average cost of $62.47 per unit while Truvada comes in at $50.04/unit.
Here at Blueberry Pharmacy, we believe in fair and transparent pricing for all. That is why when we see something going on in the market that seems fishy, we do our research! It is important for us to stay up to date on pricing, new generics, and manufacturers of brand-name medications so that we can get the lowest prices possible for our patients while also holding insurers and manufacturers accountable. Welcome to Different!
References
- Marcus JE et al. Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide for Human Immunodeficiency Virus Preexposure Prophylaxis at a Boston Community Health Center. Open Forum Infectious Diseases 8:372, 2021. https://doi.org/10.1093/ofid/ofab372
- PeBody, R. Most switches to Descovy PrEP are probably unnecessary and some may be harmful. NAM AidsMap. https://www.aidsmap.com/news/oct-2021/most-switches-descovy-prep-are-probably-unnecessary-and-some-may-be-harmful (accessed 14 July 2022).
- Side-by-side comparison: Truvada and Descovy for PrEP. San Francisco AIDS Foundation. https://www.sfaf.org/resource-library/side-by-side-comparison-truvada-and-descovy-for-prep/ (accessed 14 July 2022).